Life Cycle Assessment of Sodium-Nickel-Chloride Batteries
Battery storage systems play a vital role in solar mini grid systems by balancing the fluctuating energy generation from variable solar radiation. However, certain battery types have environmental drawbacks due to their material composition.
The Sustainable Technologies Laboratory (STL) collaborated with a battery manufacturer to investigate the environmental impacts of Sodium-Nickel-Chloride (NaNiCl2) batteries for the use-case of a solar mini grid in Tema, Ghana. The results were publisehd at IRES-Conference 2022:
Nikolic, M., Schelte, N., Velenderic, M., Adjei, F., Severengiz, S., “Life Cycle Assessment of Sodium-Nickel-Chloride Batteries”, IRES 2022 – 16th International Renewable Energy Storage Conference (IRES), Düsseldorf, 20-22 September 2022. DOI: https://doi.org/10.2991/978-94-6463-156-2_23
The Life Cycle Analysis (LCA) discovered that NaNiCl2 batteries could decrease their impact on global warming by up to 71% compared to lithium-ion (li-ion) batteries.
For more information about solar mini grids systems in Ghana, refer to the project GH2GH.
State of the Art Energy Systems
Mini grids are decentralized energy systems that can fulfill the electricity demand of local, off-grid communities [1,2], which is of particular interest in the Sub-Saharan Africa region [3]. Battery storage systems allow energy to be stored temporarily, balancing fluctuations in renewable energy generation and ensuring that the demand can be met even with changing weather conditions [4].
Li-ion and lead-acid batteries are the most widespread battery technologies. Li-ion batteries require toxic and rare earth materials. Toxic gas emissions, when damaged, pose risks to human health [5-7]. They are also sensitive to large temperature fluctuations and high heat, which leads to a shorter lifespan [8,9]. In Ghana, Lead-acid batteries, remain the dominant battery type. However, managing their end-of-life (EoL) is complicated due to increased air pollution in lead-acid battery recycling plants [10].
The NaNiCl2 battery is also known as the ZEBRA (Zero Emission Battery Research Activities) battery [11]. In the following, the general aspects of NaNiCl2 batteries are summarized:
Advantages of NaNiCl2 batteries:
- No electrochemical self-discharge due to the ceramic electrolyte [12]
- High tolerance for overcharging and deep discharging [12]
- Less expensive and rare material compared to alternative battery types [13]
- Non-flammable, safe, and recyclable composition [14]
- Material composition enables low-cost and small-scale production
- Both discharge and charge operations are minimally affected by external temperatures, according to the manufacturers
Disadvantages of NaNiCl2 batteries:
- Production capacities are not scaled-up yet, resulting in currently higher costs
- Not suitable for all applications, such as fast charging, because their charging current is limited by the endothermic charging reaction
Methodology and Assessment
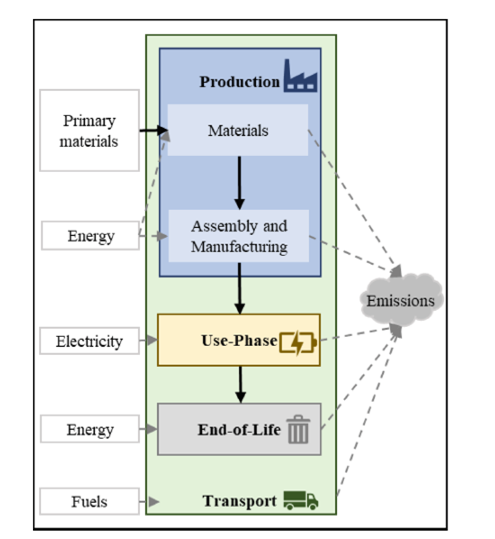
Considering the benefits and downsides of NaNiCl2 batteries, STL researchers aimed to assess their ecological impact by conducting a Life Cycle. LCA is a highly standardized method applied to assess the environmental impact of production systems by quantifying emissions as well as energy and material flows overall life cycle phases: resource and material production, manufacturing use-phase, End-of-Life (EoL), and transport, see Fig. 1. The obtained emissions and flows are then transformed to so-called impact categories
The recent study focuses on comparing NaNiCl2 batteries with alternative types, such as li-ion and lead-acid batteries, based on impacts on global warming (GWP). The calculated GWP is allocated to the “functional unit” of one 1 kWh consumed. That means the overall life-cycle GWP is divided by the amount of energy stored over the battery’s lifetime. This has the advantage of making different battery types comparable and it possible to consider parameters like efficiency and lifetime.
The analysed battery model has a usable capacity of 41 kilowatt-hours (kWh) and is composed of 140 tubular-battery cells. As NaNiCl2 is not widespread, data on EoL treatment and battery lifetime are unavailable. Moreover, EoL treatment is highly uncertain in Ghana due to the lack of an established recycling infrastructure [15]. To account for this uncertainty, the authors made a distinction between scenario 1, which assumes ideal conditions, resulting in a longer lifetime and scenario 2, which assumes a more conservative approach [12]. For EoL treatment, there are two different scenarios, A and B. Scenario A assumes the batteries are shredded, and scenario B assumes that battery materials (nickel, steel, and silicon dioxide) are recycled.
Results
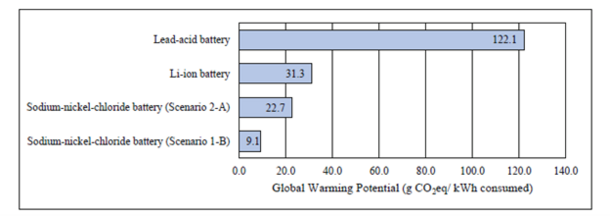
From the results of the study, shown in Figure 2., the GWP from NaNiCl2 batteries ranges from 9.1 to 22.7 g CO2eq per kWh discharged and consumed, depending on the use-phase and EoL scenario. Therefore, emissions are significantly lower than lithium-ion batteries, which emit 31.3 g CO2eq, and lead-acid batteries, which emit 122.1 g CO2eq. Implementing NaNiCl2 batteries in the mini grid configuration reduces the overall GWP by 32% compared to a configuration using a mix of NaNiCl2, lead-acid, and lithium-ion batteries.
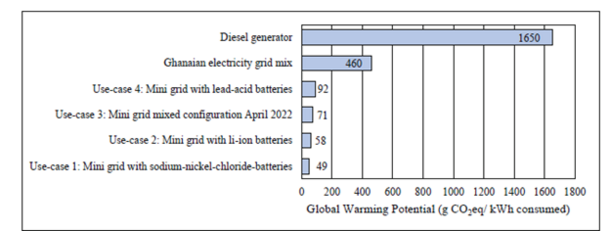
Comparing the GWP to the Ghanaian grid mix and the diesel generator, all mini grid configurations achieve a significant emission reduction, as depicted in Figure 3. GHG emissions from a mini grid based solely on NaNiCl2 batteries are reduced by 89% in comparison to the Ghanaian grid and by 97% compared to the diesel generator. Implementing the mixed mini grid configuration, GWP is still reduced by 84% compared to the grid mix and by 96% compared to a diesel generator.
Conclusion
In the introduced study, the GWP of NaNiCl2 batteries is 9.1 g CO2eq per kWh consumed in the best case, when the battery has a long lifetime of 4,500 charging cycles and nickel, steel, and silicon dioxide are recycled at EoL. Overall, NaNiCl2 batteries could decrease the GWP by up to 93% compared to lead-acid and up to 71% compared to li-ion batteries. However, to validate their environmental advantage compared to alternative research types, further research should focus on their environmental impact in categories such as depletion of resources as well as human and ecotoxicity.
References
- “The Role of Energy Storage for Mini-Grid Stabilization,” IEA-PVPS. iea-pvps.org/key-topics/the-role-of-energy-storage-for-mini-grid-stabilization/(accessed Jul. 28, 2022).
- “Off-grid renewable energy systems: Status and methodological issues,”/publications/2015/Feb/Off-grid-renewable-energy-systems-Status-and-methodologicalissues. www.irena.org/publications/2015/Feb/Off-grid-renewable-energy-systems-Status-and-methodological-issues(accessed Jul. 28, 2022).
- D. A. Quansah, M. S. Adaramola, and L. D. Mensah, “Solar Photovoltaics in Sub-Saharan Africa – Addressing Barriers, Unlocking Potential,” Energy Procedia, vol. 106, pp. 97–110, Dec. 2016, doi: doi.org/10.1016/j.egypro.2016.12.108
- S. AMDC Energy Limited, “Battery Energy Storage Systems.” www.amdcenergy.com/markets-services/energy-strorage/battery-energy-storage-systems.html(accessed Aug. 08, 2022).
- A. Nedjalkov et al., “Toxic Gas Emissions from Damaged Lithium Ion Batteries—Analysis and Safety Enhancement Solution,” Batteries, vol. 2, no. 1, Art. no. 1, Mar. 2016, doi: doi.org/10.3390/batteries2010005.
- M. Bilharz, “Lithium-Batterien und Lithium-Ionen-Akkus,” Umweltbundesamt, Jan. 22, 2015. www.umweltbundesamt.de/umwelttipps-fuer-den-alltag/elektrogeraete/lithium-batterien-lithium-ionen-akkus(accessed Jul. 28, 2022).
- Y. Chen et al., “A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards,” J. Energy Chem., vol. 59, pp. 83–99, Aug. 2021, doi: doi.org/10.1016/j.jechem.2020.10.017.
- A. Väyrynen and J. Salminen, “Lithium ion battery production,” J. Chem. Thermodyn., vol. 46, pp. 80–85, Mar. 2012, doi: doi.org/10.1016/j.jct.2011.09.005.
- J. Hou, M. Yang, D. Wang, and J. Zhang, “Fundamentals and Challenges of Lithium Ion Batteries at Temperatures between −40 and 60 °C,” Adv. Energy Mater., vol. 10, no. 18, p. 1904152, 2020, doi: doi.org/10.1002/aenm.201904152.
- I. A. Bergdahl and S. Skerfving, “Chapter 19 - Lead.” reader.elsevier.com/reader/sd/pii/B9780128229460000362(accessed Jul. 28, 2022).
- H. Sakaebe, “ZEBRA Batteries,” in Encyclopedia of Applied Electrochemistry, G. Kreysa, K. Ota, and R. F. Savinell, Eds. New York, NY: Springer, 2014, pp. 2165–2169. doi: doi.org/10.1007/978-1-4419-6996-5_437.
- EASE, “Sodium-Nickel-Chloride Battery,” EASE Storage, 2022. ease-storage.eu/energy-storage/technologies/(accessed Jul. 29, 2022).
- R. Weidl, M. Schulz, M. Hofacker, H. Dohndorf, and M. Stelter, “Low cost, ceramic battery components and cell design,” Freiberg, Germany, 2016, p. 020004. doi: doi.org/10.1063/1.4961896.
- R. Manzoni, “Sodium Nickel Chloride batteries in transportation applications,” in 2015 International Conference on Electrical Systems for Aircraft, Railway, Ship Propulsion and Road Vehicles (ESARS), Mar. 2015, pp. 1–6. doi: doi.org/10.1109/ESARS.2015.7101491.
- K. Owusu-Sekyere, A. Batteiger, R. Afoblikame, G. Hafner, and M. Kranert, “Assessing data in the informal e-waste sector: The Agbogbloshie Scrapyard,” Waste Manag., vol. 139, pp. 158–167, Feb. 2022, doi: doi.org/10.1016/j.wasman.2021.12.026.
- A. K. Stinder, S. Finke, M. Vendeleric, and S. Severengiz, “A generic GHG-LCA model of a smart mini grid for decision making using the example of the Don Bosco mini grid in Tema, Ghana,” Procedia CIRP, vol. 105, pp. 776–781, Jan. 2022, doi: doi.org/10.1016/j.procir.2022.02.129.
- Institute for Global Environmental Strategies, “List of Grid Emission Factors version 10.10,” 2021. pub.iges.or.jp/pub/iges-list-grid-emission-factors
- Kristina Juhrich, “Climate Change. CO2 Emission Factors for Fossil Fuels,” German Environment Agency (UBA), 2016.
Acknowledgments
Research was funded by quality improvement funds of Bochum University of Applied Sciences. Data sets used in this research were funded by the German Federal Ministry of Education and Research within the project SCiSusMob, grant number 13FH0I73IA. Data on battery characteristics and manufacturing were kindly provided by the company Battery Consult. We thank Brent Hendrickx for assistance with literature recommendations for the life cycle inventory of the battery